How To Find Homo And Lumo On Orca. The key difference between homo and lumo is that the homo donates electrons whereas the lumo receives electrons. Homo stands for highest occupied molecular orbital, and lumo stands for lowest unoccupied molecular orbital.don't be confused though, because the lumo is higher in energy than the homo.
That means they are closest in energy out of all orbitals in the. Using semiempirical am1 method, 615 benzocatafusenes were studied (29 normal and 586 branched). With α = 1, β = 4.8 ev); A simple model for the calculation of homo and lumo energy levels of benzocatafusenes (i.e., molecules that are only constituted by mutually condensed benzene rings and without interior carbon atoms, which belong to three benzene rings) is presented. Homo stands for highest occupied molecular orbital, and lumo stands for lowest unoccupied molecular orbital.don't be confused though, because the lumo is higher in energy than the homo.

Conjugation in alkadienes and allylic systems.
1) identify the possible homo's and lumo's 2) select the likely nucleophile and electrophile from these two species 3) show how these species will react using curved arrows 4) predict the immediate product of that reaction The key difference between homo and lumo is that the homo donates electrons whereas the lumo receives electrons. The data were taken from table 1. The terms homo and lumo are under the subtopic molecular orbital theory in general chemistry. How to mix homo and lumo in orca? Sometimes the lumo is unusually low , like the one indicated in red on the far. If energies are converted to ev from hatree, then homo and lumo will be 27.86 ev and 30.52 ev, i think these are unrealistic values for drug molecules. In orca, after the optimization→open the file.out → search for orbital energies → scroll down till occ = 0. For example, the electrophilic (homo) frontier density of surface of formaldehyde is blue (maximum value) around the oxygen. For example, in the case of a bromine molecule (br2), the bond is broken by exposing it to light energy. The lumo is the lowest energy place to put or excite an electron. Based on cyclic voltammetry results, pcbe shows e homo = 5.87 ev, e gap = 1.96 ev and e lumo = 3.91. The homo is the highest energy mo that has any electrons in it.
If energies are converted to ev from hatree, then homo and lumo will be 27.86 ev and 30.52 ev, i think these are unrealistic values for drug molecules. Under the orca/propenal/ directory on your desktop, you should be able to find a file named propenal.xyz, which contains the In short, the oxidation potential involves removing an electron from the molecule, and the lowest ionization potential should come from the homo. However, the energy that i get using orca is much higher in value than what is reported in. (d corr) a combination of cv and the optical gap, homo calculated from eq.

Accepted january 15, 2018) keywords:
Homo stands for highest occupied molecular orbital, and lumo stands for lowest unoccupied molecular orbital.don't be confused though, because the lumo is higher in energy than the homo. That means they are closest in energy out of all orbitals in the. In orca, after the optimization→open the file.out → search for orbital energies → scroll down till occ = 0. The correspondence to the homo energy is based on an interpretation of koopmans' theorem. Hi, when opening the log of gamess results in avogadro i see that the energy of the orbitals (homo, lumo) given in ev are completely wrong, e.g. The lumo is the next highest energy orbital (it will be empty). Sometimes the lumo is unusually low , like the one indicated in red on the far. 1) identify the possible homo's and lumo's 2) select the likely nucleophile and electrophile from these two species 3) show how these species will react using curved arrows 4) predict the immediate product of that reaction Using semiempirical am1 method, 615 benzocatafusenes were studied (29 normal and 586 branched). However, the energy that i get using orca is much higher in value than what is reported in. From dft, highest occupied molecular orbital (homo) and lowest unoccupied molecular orbital (lumo) energy levels of frontier orbital were obtained. (d corr) a combination of cv and the optical gap, homo calculated from eq. Based on cyclic voltammetry results, pcbe shows e homo = 5.87 ev, e gap = 1.96 ev and e lumo = 3.91.
1) identify the possible homo's and lumo's 2) select the likely nucleophile and electrophile from these two species 3) show how these species will react using curved arrows 4) predict the immediate product of that reaction Every molecule has a highest occupied molecular orbital (homo) and a lowest unoccupied molecular orbital (lumo). (d corr) a combination of cv and the optical gap, homo calculated from eq. For example, in the case of a bromine molecule (br2), the bond is broken by exposing it to light energy. The last orbital with occ=1 is your homo.
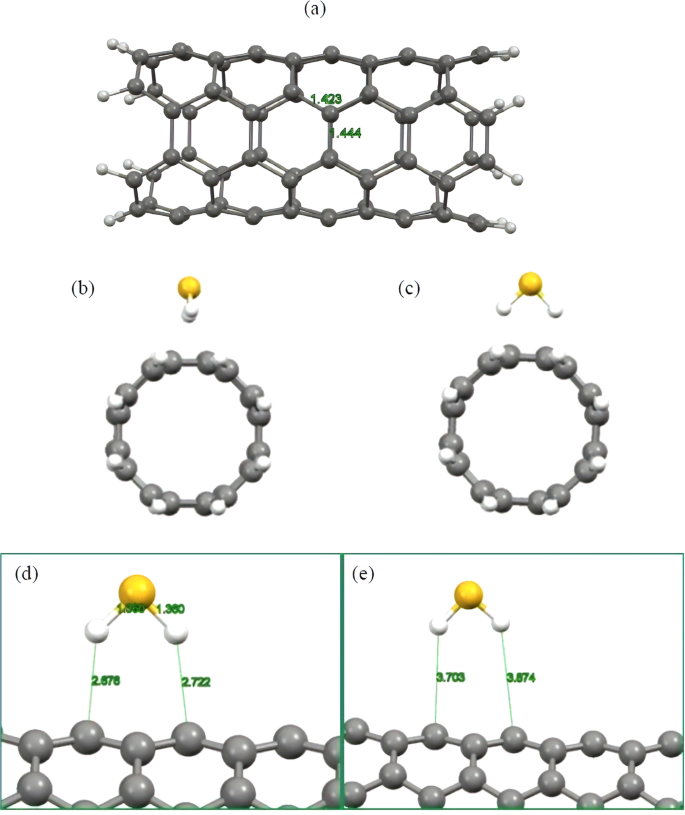
The molecule has several states both above and below the fermi level, which by itself is a point of confusion for me because from the band.
Accepted january 15, 2018) keywords: From dft, highest occupied molecular orbital (homo) and lowest unoccupied molecular orbital (lumo) energy levels of frontier orbital were obtained. So what that means is that our homo orbital is pi 1, so we would say that the homo contains two electrons, it's pi 1 and that means that my lumo orbital is pi 2 and has zero electrons, that's it, so homo is the highest one that still has electrons, which in this case it's the only one, because it's the only one that exists and lumo has no. However, the energy that i get using orca is much higher in value than what is reported in. 1) identify the possible homo's and lumo's 2) select the likely nucleophile and electrophile from these two species 3) show how these species will react using curved arrows 4) predict the immediate product of that reaction Can someone please suggest me where to exactly find the energy of homo and lumo in the output file? Accordingly, the saddling of porphyrin core leads to the decrease of the homo/lumo gap and no change of homo−1/lumo. Based on cyclic voltammetry results, pcbe shows e homo = 5.87 ev, e gap = 1.96 ev and e lumo = 3.91. Using semiempirical am1 method, 615 benzocatafusenes were studied (29 normal and 586 branched). Under the orca/propenal/ directory on your desktop, you should be able to find a file named propenal.xyz, which contains the Enjoy the videos and music you love, upload original content, and share it all with friends, family, and the world on youtube. With α = 1, β = 4.8 ev); Homo is the highest occupied molecular orbital whereas lumo is the lowest unoccupied molecular orbital.


Tidak ada komentar:
Posting Komentar